OLIGONUCLEOTIDE BASED THERAPEUTICS
Treatment of genetic neuromuscular diseases
by using its tricyclo-DNA molecular technology
ABOUT US
Synthena’s mission is to develop first in class drugs in the field of Myopathy with a focus on the Duchenne Muscular Dystrophy disease, based on its proprietary tricyclo-DNA technology.
Its proprietary tricyclo-DNA technology offers broad advantages over state of the art oligonucleotide chemistries for new RNA intervention strategies like splice switching, and other antisense approaches. Its most advanced preclinical drug development program focuses on Duchenne Muscular Dystrophy, a genetic disorder which affects 1 in 3,500 boys and which leads to death in early adulthood.
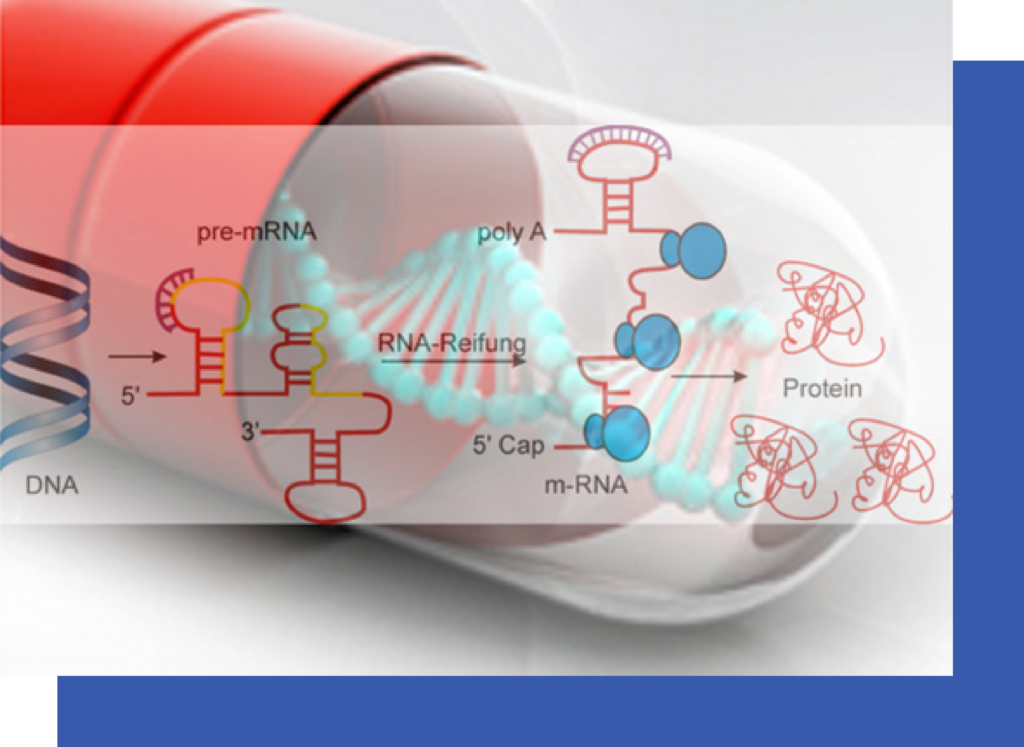
Modulation of RNA for therapeutic purposes holds the promise of opening up entirely new concepts for pharmaceutical intervention, however, current oligonucleotide chemistries to modulate RNA do not meet the requirements for efficient delivery, pharmacokinetics and tolerability.
LEADERSHIP
MANAGEMENT TEAM - DIRECTORS & FOUNDERS

Pascal Ferré
Pascal Ferré is the parent of a boy with Duchenne muscular dystrophy. He is a chartered accountant and ...
MORE
To find a cure for this disease, he helped found SYNTHENA AG with other families, DPP France, the Association Monégasque contre les Myopathies and researchers.
TcDNA, developed and patented by SYNTHENA AG, is currently used by other companies to design AONs targeting Duchenne disease and many other diseases of genetic origin.
LESS

Terrence Partridge
Chairman
Terrence Partridge, a renowned researcher in the field of myology, the science of muscle. He is a professor at...
MORE
LESS

Luis Garcia
Luis Garcia received his PhD in 1989 at the University Paris 7 (Diderot) in the laboratory of Michel Fardeau (INSERM U153/UA614 CNRS) on the role of the L-type Ca2+ channel in excitation-contraction coupling in skeletal muscle. After a Post-doctoral experience...
MORE
LESS

Markus Ruegg
Markus Rüegg is a Swiss neurobiologist and professor at the Biozentrum of the University of Basel. Markus Rüegg studied biochemistry at the University of Zurich and graduated with a PhD in the field of Neurobiology. In 1989...
MORE
Rüegg studies the molecular principles that are essential for the development and the maintenance of the neuromuscular system. The major achievements of his earlier work include the isolation and functional characterization of proteins involved in axonal pathfinding,[7] synapse formation[8][9][10][11] and in mediating changes in synapse structure upon learning.[12] Furthermore, for the last 20 years his laboratory is interested in understanding the disease mechanisms involved in congenital muscular dystrophies and recent findings of his laboratory have led to the development of a novel therapeutic strategy.[13][4] In addition, his research group has recently demonstrated that the multi-protein complex mTORC1 is essential for muscle homeostasis and is associated to precocious sarcopenia, the loss of muscle mass and function at advanced age.[14][15] This knowledge may help to counteract pathological muscle degradation and to develop new therapeutic strategies.
LESS
SCIENCE
Antisense Technology
Synthena AG is a development stage biotechnology company aiming to develop and commercialize new life saving drugs for the treatment of severe neuromuscular diseases based on the modulation of RNA. Its proprietary tricyclo-DNA technology platform offers broad advantages over state of the art oligonucleotide chemistries for new RNA intervention strategies like splice switching, and other antisense approaches. Its most advanced preclinical drug development program focuses on Duchenne Muscular Dystrophy, a genetic disorder which affects 1/3,500 boys and which leads to death in early adulthood.
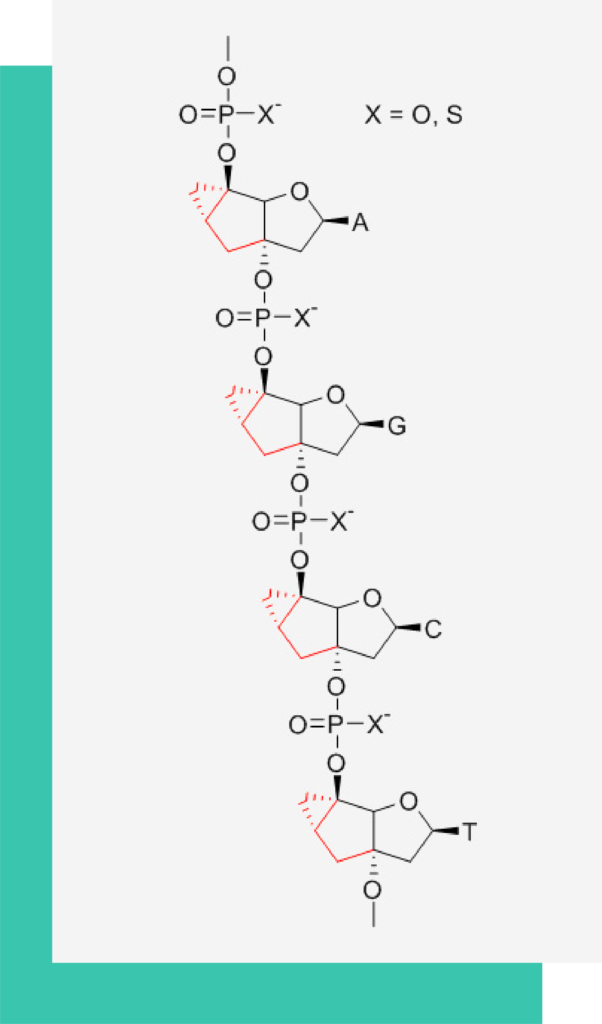
Much as LNA, tricyclo(tc)-DNA has been designed as a conformationally constrained oligonucleotide analogue. Chemically, tc-DNA deviates from natural DNA by three additional C-atoms between C(5’) and C(3’). These chemical modifications change the properties of natural oligodeoxynucleotides in the following way:
Increased RNA affinity by 2 – 4°C / modification
Increased hydrophobicity
Increased stability towards nucleolytic degradation for both, phosphate or thiophosphate internucleoside linkages
Inability to elicit RNaseH activity
Tc-oligonucleotides are manufactured in the same way as natural or LNA- oligonucleotides via solid phase phosphoramidite chemistry.
Unlike LNA, fully modified tc-oligonucleotides in the lenght range of 11-25 nucleotides can easily be prepared and produce potent antisense effects.
Due to their properties tc-oligonucleotides are particularly suited for biological and therapeutic applications where high target affinity and biostability is required and where the mechanism of action does not rely on RNaseH activity.
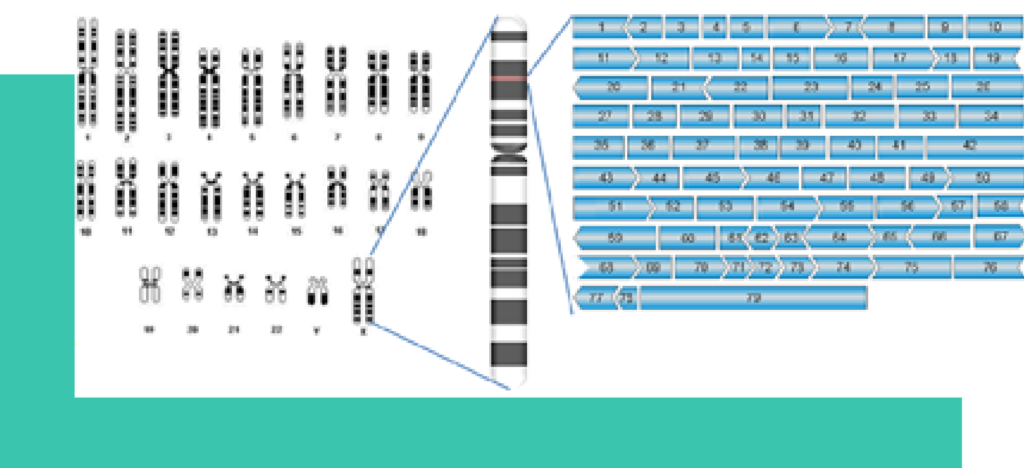
Duchenne muscular dystrophy (DMD) is the consequence of a X-chromosome linked genetic defect, caused by a mutation in the dystrophin gene. Dystrophin is the largest known human gene consisting of 79 exons interrupted by 78 introns. Mutations can lead to exon skipping during splicing which in turn leads to misaligned mRNA that produces unfunctional protein. The incidence rate of the disease is ca 1 out of 3500 newborn males. The majority of mutations are spontaneous and not hereditary.
Dystrophin binds to the intracellular actin filaments and links via the trans-membrane sarcoglycans to the extracellular matrix. It is essential for muscle function.
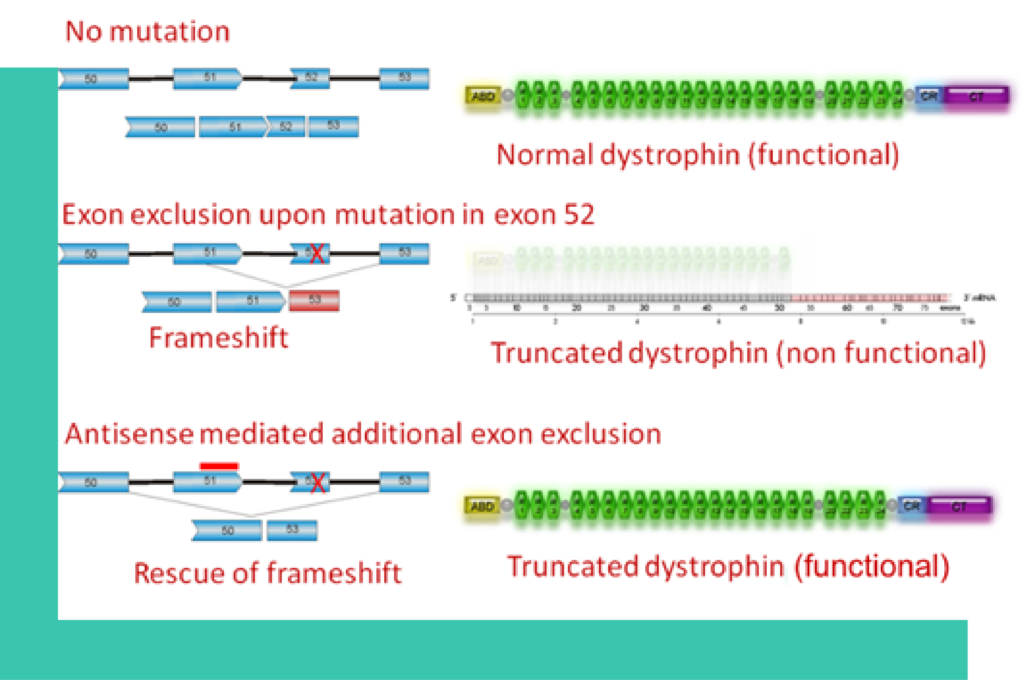
Classical mutations (e.g. in exon 52) cause exon 52 skipping during splicing which leads to a frame shifted mRNA that produces unfunctional dystrophin. Additional skipping of exon 51 induced by an antisense oligonucleotide restores the reading frame and leads to a slightly shortened but functional dystrophin variant.
- R. Steffens, C. Leumann, “Tricyclo-DNA: A phosphodiester-backbone based DNA analog exhibiting strong complementary base-pairing properties”, J. Am. Chem. Soc.1997, 119, 11548-11549.
- R. Steffens, C. Leumann, “Nucleic-Acid Analogs with Constraint Conformational Flexibility in the Sugar-Phosphate Backbone ‘Tricyclo-DNA’, Part 1, Preparation of [(3’S,5’R,6’R)-2′-Deoxy-3′,5′-ethano-5′,6′-methano-beta-D-ribofuranosyl]thymine and -Adenine and the Corresponding Phosphoramidites for Oligonucleotide Synthesis, Helv. Chim. Acta1997, 80, 2426-2439.
- R. Steffens, C. Leumann, “Synthesis, Thermodynamic and Biophysical Properties of Tricyclo-DNA”,J. Am. Chem. Soc., 1999, 120, 3249-3255.
- D. Renneberg, C. J. Leumann: Watson-Crick base-pairing properties of tricyclo-DNA, J. Am. Chem. Soc., 2002, 124, 5993-6002.
- D. Renneberg, E. Bouliong, U. Reber, D. Schümperli, C. J. Leumann: Biological and antisense properties of tricyclo-DNA, Nucleic Acids Res., 2002, 30, 2751-2757.
- D. Ittig, S. Liu, D. Renneberg, D. Schümperli, C. J. Leumann, Nuclear antisense effects in cyclophilin A pre-mRNA splicing by oligonucleotides: a comparison of tricyclo-DNA with LNA, Nucleic Acids Res.2004, 32, 346-353.
- S. P. Scheidegger, C. J. Leumann, Synthesis and Pairing Properties of a-tricyclo-DNA, Chem. Eur. J.2006, 12, 8014-8023.
- G. Ivanova, S. Reigadas, D. Ittig, A. Arzumanov, M.-L. Andreola, C. Leumann, J.J. Toulmé, M. J. Gait, Tricyclo-DNA Containing Oligonucleotides as Steric Block Inhibitors of HIV-1 Tat-Dependent TransActivation and HIV-1 Infectivity, Oligonucleotides, 2007, 17, 54-65.
- P. S. Pallan, D. Ittig, A. Héroux, Z. Wawrzak, C. J. Leumann, M. Egli*, Crystal Structure of Tricyclo-DNA: An Unusual Compensatory Change of Two Adjacent Backbone Torsion Angles, Chem. Commun. 2008, 883-885.
- D. Ittig, S. Luisier, J. Weiler, D. Schümperli, C. J. Leumann, Improving gene silencing of siRNAs via tricyclo-DNA modification, Artificial DNA, PNA & XNA, 2010, 1, 9-16
- D. Ittig, A-B. Gerber, C. J. Leumann, Position dependent effects on stability in tricyclo-DNA modified oligonucleotide duplexes, Nucleic Acids Res. 2011, 39, 373-380.
- S. Murray, D. Ittig, E. Koller, A. Berdeja, A. Chappell, T. P. Prakash, M. Norrbom, E. E. Swayze, C. J. Leumann, P. P. Seth, TricycloDNA modified oligo-2′-deoxyribonucleotides reduce scavenger receptor B1 mRNA in hepatic and extra-hepatic tissues – a comparative study of oligonucleotide length, design and chemistry, Nucleic Acids Res. 2012, 40, 6135-6143.
- J. Lietard, C. J. Leumann, Synthesis, Pairing, and Cellular Uptake Properties of C(6′)-Functionalized Tricyclo-DNA, J. Org. Chem. 2012, 77, 4566-4577.
- A. Goyenvalle, G. Griffith, A. Babbs, S.E. Andaloussi, K. Ezzat, A. Avril, B. Dugovič, R. Chaussenot, A. Ferry, T. Voit, H. Amthor, C. Bühr, S. Schürch, M.J. Wood, K.E. Davies, C. Vaillend, C.J. Leumann, L. Garcia, Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers, Nature medicine. 2015, 21, 270-275.
- K. Ahmed, Y. Aoki, T. Koo, G. McClorey, L. Benner, A. Coenen-Stass, L. O’Donovan, T. Lehto, A. Garciaguerra, J.Z. Nordin, A.F. Saleh, M.A. Behlke, J. Morris, A. Goyenvalle, B. Dugovič, C.J. Leumann, S. Gordon, M.J. Gait, S. El-Andaloussi, M.J. Wood, Self-assembly into nanoparticles is essential for receptor mediated uptake of therapeutic antisense oligonucleotides, Nano letters. 2015, 15, 4364-4374.
- A. Goyenvalle, C. Leumann, L Garcia, Therapeutic Potential of Tricyclo-DNA antisense oligonucleotides, Journal of Neuromuscular Diseases, 2016, 3(2), 157-167.
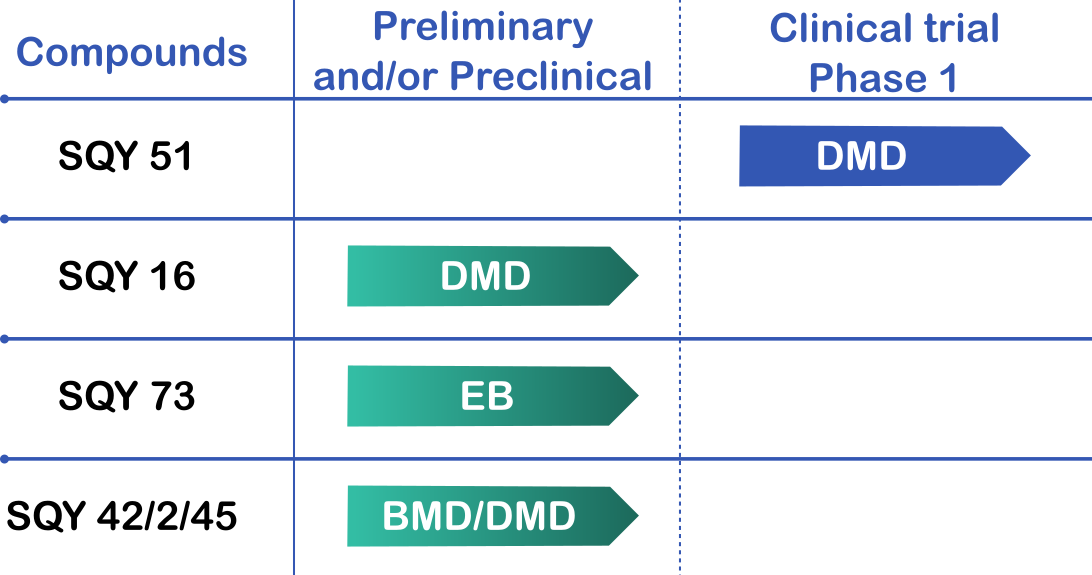
PIPELINE